Key learning points
In a reversible reaction the productA chemical which is made in a chemical reaction. Products are written on the right of a chemical equation, after the arrow (→). can react to turn back into the reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow →.
A dynamic equilibrium is formed in a reversible reaction when the rates of the forward and reverse reaction are equal and the amounts of the reactants and the products remains constant.
Le Châtelier’s principleThis states that if a change is made to the conditions of a system at equilibrium, then the position of equilibrium moves to oppose that change in conditions. can be used to predict changes to the position of an equilibrium. This is useful when explaining the conditions used in the Haber processThe industrial chemical process that makes ammonia by reacting nitrogen and hydrogen together. (making ammonia).
What are reversible reactions?
In many chemical reactions the reactants are converted into products and then the reaction stops.
For example:
2Mg + O2 → 2MgO
In this reaction, once the magnesium oxide has formed, it cannot go back to reform magnesium and oxygen.
This is a non-reversible reaction.
Other chemical reactions are reversible reactions.
In these reactions, the products, once formed, can change back into the original reactants.
For example:
N2 + 3H2 ⇌ 2NH3
In this reaction, once the ammonia (NH3) has formed, it can ‘go back’ to reform nitrogen and hydrogen.
Reversible reactions are shown with reversible arrows (⇌).
The reversible arrow has two ‘half-arrows,’ one pointing in each direction.
What is an example of a reversible reaction?
Hydrated copper(II) sulfate is blue in appearance.
When it is heated, the water of crystallisationWater that is chemically bonded into a crystal structure. is removed, leaving anhydrous copper(II) sulfate which is white in appearance.
Equation: hydrated copper(II) sulfate ⇌ anhydrous copper(II) sulfate + water
CuSO4.5H2O(s) ⇌ CuSO4(s) + 5H2O(l)
This reaction is reversible, as the hydrated copper(II) sulfate is reformed if water is added to anhydrous copper(II) sulfate.
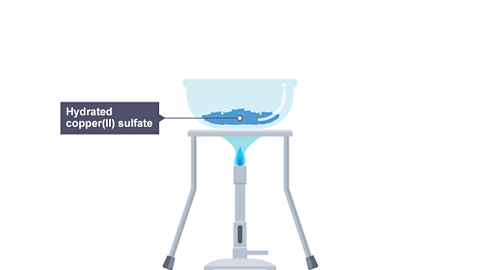
Image caption, 1. A Bunsen burner is used to heat an evaporating basin containing hydrated copper(II) sulfate.
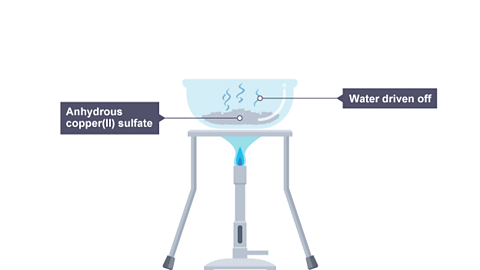
Image caption, 2. Water is driven off, leaving anhydrous copper(II) sulfate.
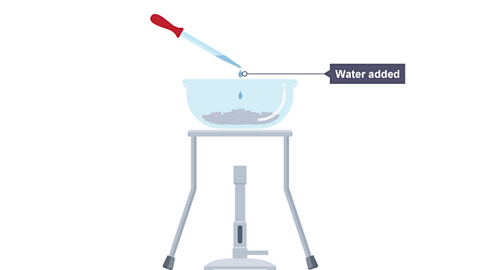
Image caption, 3. The Bunsen burner is turned off and water is added using a pipette.
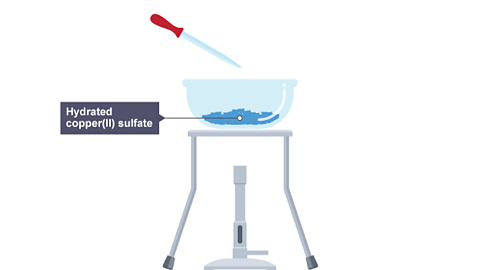
Image caption, 4. The evaporating basin now contains hydrated copper(II) sulfate again.
1 of 4
What is dynamic equilibrium?
When a reversible reaction happens in a closed system (closed container) , a dynamic equilibriumA dynamic equilibrium is one in which the rate of the forward reaction equals the rate of the reverse reaction and the amounts of reactants and products remain constant. can be achieved.
In a dynamic equilibrium:
the rates of the forward and reverse reactions are equal.
the amounts of reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow →. and productA chemical which is made in a chemical reaction. Products are written on the right of a chemical equation, after the arrow (→). remains constant.
For example, consider a reversible reaction where gas A reacts to form a different gas B:
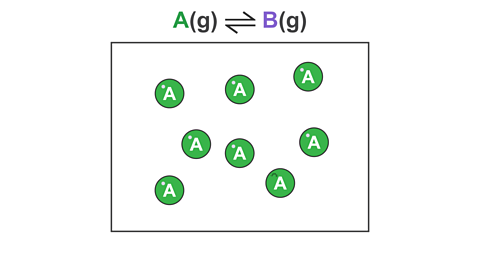
Image caption, 1. At the start of the reaction, the container contains only A.
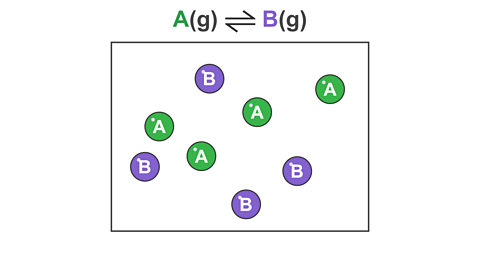
Image caption, 2. As the reaction proceeds, A is converted into B.
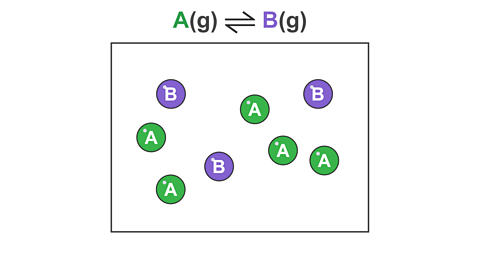
Image caption, 3. Once B has formed, it can reverse and turn back into A. Eventually the rate of the forward and reverse reactions are equal, and the amount of A and B remain fixed (even though both reactions are still occurring).
1 of 3
An everyday illustration of dynamic equilibrium is a person trying to walk up an escalator that is moving downwards.
The person appears to be stationary (not moving), but this is just because the rate of their walking up is equal to the rate the escalator is moving downwards.
How to change the position of equilibrium (Higher tier only)
The position of an equilibrium can be described as on the ‘left’ or ‘right’.
This describes how much of the reactants (on the left) and the products (on the right) are present at equilibrium.
The diagram below shows an example of an equilibrium on the left and an equilibrium on the right.
What is Le Châtelier’s principle?
The equilibrium position can be changed by altering the reaction conditions, such as by:
changing the pressure.
changing the concentration.
changing the temperature.
The effect of changing a reaction condition on the position of equilibrium can be predicted using Le Châtelier’s principle.
This states that if a change is made to the conditions of a system at equilibrium, then the position of equilibrium moves to oppose that change in conditions.
What happens when you change the concentration?
If the concentration of a reactant or product is changed in a reaction at equilibrium, the position of the equilibrium moves to oppose the change.
For example:
CH4(g) + H2O(g) ⇌ CO(g) + 3H2(g)
If the reaction is at equilibrium and the concentrationThe amount of a substance that has been dissolved in a certain amount of solution. Measured in mol/dm3 (moles per decimetre cubed). of CH4 is increased:
- The equilibrium moves to decrease the concentration of CH4.
- The forward reaction is favoured which reduces the concentration of CH4.
- The equilibrium position moves to the right.
Question
For the same equilibrium described above, what would happen to the position of the equilibrium if the concentration of CO was increased?
Answer
The equilibrium moves to decrease the concentration of CO.
The reverse reaction is favoured which reduces the concentration of CO.
The equilibrium position moves to the left.
What happens when you change the pressure?
For a reaction involving gases, the greater the number of gas particles present, the greater the pressure in the system.
If the pressure exerted on a reaction at equilibrium is changed, the position of the equilibrium moves to oppose the change in pressure.
For example:
N2(g) + 3H2(g) ⇌ 2NH3(g)
If the reaction is at equilibrium and the pressure is increased:
The equilibrium moves to decrease the pressure.
The equilibrium moves in the direction of the side of the reaction with fewer gas particles to reduce the pressure.
The equilibrium position moves to the right, as there are two gas particles on the right hand side of the equation, and 4 gas particles on the left hand side of the equation.
Question
For the same equilibrium described above, what would happen to the position of the equilibrium if the pressure was decreased?
Answer
The equilibrium position moves to the left, as there are four gas particles on the left hand side of the equation, and 2 gas particles on the right hand side of the equation.
This increases the pressure, which opposes the change that has been made.
What happens when you change the temperature?
In a reversible reaction, if the reaction is exothermicA physical change or chemical reaction that transfers energy to the surroundings. in one direction, it is endothermicA physical change or chemical reaction that absorbs energy from the surroundings. in the other direction.
If the temperature of a reaction at equilibrium is changed, the position of the equilibrium moves to oppose the change in temperature.
For example:
2SO2(g) + O2(g) ⇌ 2SO3(g) (forward reaction is exothermic)
If the reaction is at equilibrium and the temperature is increased:
The equilibrium moves to decrease the temperature.
The reverse reaction is endothermic, so this reaction is favoured to decrease the temperature.
The equilibrium moves to the left.
Question
For the same equilibrium described above, what would happen to the position of the equilibrium if the temperature was decreased?
Answer
The equilibrium moves to increase the temperature.
The forward reaction is exothermic, so this reaction is favoured to increase the temperature.
The equilibrium moves to the right.
Adding a catalyst
Adding a catalyst A substance that speeds up a chemical reaction without being used up or chemically changed. It does this by providing an alternative reaction pathway with a lower activation energy. has no effect of the position of an equilibrium as it increases the rate of the forward and reverse reactions equally.
What is the Haber process? (Higher tier only)
Ammonia is an important industrial product used to make fertilisers, explosives and dyes.
It is manufactured using the Haber process.
This involves a reversible reaction between nitrogen and hydrogen:
N2(g) + 3H2(g) ⇌ 2NH3(g)
The reaction can reach a dynamic equilibriumA dynamic equilibrium is one in which the rate of the forward reaction equals the rate of the reverse reaction and the amounts of reactants and products remain constant..
Conditions
The following conditions are used in the Haber process:
| Catalyst | Iron |
| Temperature | 450oC |
| Pressure | 200 atm |
The iron catalyst is used to increase the rate of the reaction.
The temperature and pressure conditions used are selected to give a compromise between the yield of ammonia, the rate of the reaction, and the cost of the process.
Temperature
The reaction between nitrogen and hydrogen is exothermic in the forward direction.
Therefore a low temperature would cause the equilibrium to move to the right and increase the yieldThe amount of product obtained. of ammonia.
However, a low temperature would cause a very slow rate of reaction.
The temperature used (450oC) allows a compromise between yield and rate.
Pressure
There are fewer gas particles on the right hand side of the reaction.
Therefore a high pressure would cause the equilibrium to move to the right and increase the yield of ammonia.
However, a high pressure is expensive to implement and would increase the risk of explosion.
The pressure used (200 atm) allows a compromise between yield and cost.
WATCH: Equilibrium
Test your knowledge
More on Unit 2: Further chemical reactions, rates and equilibrium, calculations and organic chemistry
Find out more by working through a topic
- count5 of 9

- count6 of 9

- count7 of 9

- count8 of 9